Glaciers are usually perceived as gorgeous, if rather dangerous, white and blue components of polar and high-mountain landscapes. They store fresh water, reflect solar radiation and shape the functioning of marine and land ecosystems. Unfortunately, during the age of man, known as the Anthropocene, they are also becoming dumping grounds, repositories of pollutants, which our civilization generates as it develops.
Many of these pollutants can be spotted without the use of sophisticated laboratory techniques. Black carbon (formed through the incomplete combustion of biofuels), litter (left behind by tourists), battle remains (such as lead bullets in the Alps) or scientific equipment (scattered around by high winds) are all visible with the naked eye. There is, however, much more to the problem, as the world’s glaciers have become polluted with a lot that can’t be seen.
Radioactivity
The term “radioactivity” stirs up much emotion. We all know from school – and from cinema productions – that radioactive waste or, more precisely, the radiation emitted by the waste causes mutations and cancer. There are those who believe it might also give you superpowers, yet this kind of effect has only been seen in films. Let us, however, start at the beginning. Isotopes are variants of the same chemical element that differ in terms of the number of neutrons in their nuclei. They can be stable, in which case their nuclei don’t decay, or unstable, which means they do decay, releasing energy (radiation) in the process. Unstable isotopes are referred to as radioactive elements or radioisotopes and have accompanied life on Earth from the very beginning. Forms like the unstable carbon isotope 14C can be found inside our bodies and they don’t turn us into superheroes or make us ill. The energy released during their decay makes no significant damage to our cells or tissues. Some scientists make bold claims that small doses of radiation were, in fact, beneficial for the development and evolution of life on Earth. Apart from natural radioisotopes, however, there is a whole range of grim synthetic ones. They form as a result of accidents at nuclear power plants (Chernobyl, Fukushima), nuclear weapons tests (Nevada, Novaya Zemlya) or even satellite disintegration in the atmosphere. These radioactive consequences of human activity include, among others, the synthetic isotopes of caesium, plutonium or americium.
Are they dangerous to humans? Very much so. We’ve all heard of acute radiation syndrome caused specifically by prolonged exposure to a harmful radioisotope. Radioisotopes emit alpha, beta or gamma radiation, which has the ability to ionize matter or – to put it more simply – to tear electrons away from atomic nuclei. Particles released from a nuclei lose their energy by colliding into human tissues, which has a negative effect on our health. To protect our bodies from alpha radiation we need as little as a sheet of paper or a bit of distance between ourselves and the emitter. With gamma radiation we would need a thick lead wall or a few metres of concrete! The plutonium isotope 239Pu, which emits alpha radiation, can cause lung cancer; the strontium isotope 90Sr, which emits beta radiation, causes bone cancer, while the isotope of caesium 137Cs, which emits gamma radiation, has not only strong carcinogenic potential, but causes a whole range of other serious health issues. The available research indicates that exposure to caesium will severely damage our lungs and pancreas, and negatively affect our circulatory system.
It is generally assumed that the major source of radioactive caesium and other synthetic radioisotopes is accidents at nuclear plants, but the bulk of these particles was, in fact, released during Cold War nuclear tests. Either way, what does all this have to do with glaciers?
Glaciers and synthetic radioisotopes
Even glaciers did not escape radioactive fallout. Quite the opposite. It turned out that they effectively capture radioactive contaminants, which means their concentration in glacier ice is a reliable indicator of changes taking place in mountain and polar ecosystems. Glacier surface is inhabited by a range of organisms, which are too tiny to see with the naked eye, but still play an important role in contaminant accumulation. More about these organisms has already been said here. Dark sediment often covering glacier surface is not only dust blown over from nearby moraines and mountain slopes, but also pigmented organisms, such as cyanobacteria and ice algae, which take part in the production of dark organic matter. The matter is made up of various life forms, including cold-loving heterotrophic bacteria and fungi. Cyanobacteria, for instance, produce extracellular polymeric substances (or, to put it more simply, layers of sticky mucus), which serve a cryoprotective function and shield cyanobacteria from high doses of UV radiation. At the same time, though, the substances act as glue, capturing windblown dust particles and various contaminants. Mineral sediment combined with living organisms and organic matter is known as cryoconite. Deposits of cryoconite are dark in colour and absorb solar radiation, which melts the ice beneath them and leads to the formation of the so-called cryoconite holes. Living organisms and organic matter making up cryoconite on glacier surface and at the bottom of cryoconite holes accumulate pollutants exceptionally well. And this, as it turns out, applies also to synthetic radioisotopes.
Radioactivity is measured in becquerels (Bq), a unit named after Henri Becquerel. Radionuclide (or synthetic radioisotope) activity in dry tundra soil exceeds 100 Bq/kg 137Cs, while in the soil of glacier forefields in Svalbard it goes beyond 3000 Bq/kg. But most worrying of all is the concentration of radionuclides in cryoconite found on a glacier in Norway: a staggering 20 000 Bq/kg 137Cs and over!
Polish research into glaciers’ radioactivity
The history of research into the radioactivity of glaciers is strictly connected with Polish research work in polar and mountain regions. First investigations into the presence of caesium isotopes were conducted by Polish radiologist Zbigniew Jaworowski, who studied their concentration in glacier ice in Antarctica, South America, Africa, Europe and the Arctic. The isotopes came from nuclear weapons tests and the research, carried out in the 1970s, was both pioneering and impressively large scale. Contrary to what was generally the case in socialist Poland, Jaworowski’s results were published in English and thus circulated internationally.
Nowadays, the leading specialist in glacier contamination with synthetic radionuclides is Professor Edyta Łokas from the Institute of Nuclear Physics of the Polish Academy of Sciences in Kraków, who runs international research projects on the topic. Radionuclide concentration measurements performed by the Institute involve glaciers from across the globe. Instead of ice, however, Polish scientists focus on cryoconite, which proved to be an excellent matrix for the study of radionuclide concentration and distribution in polar and high-mountain regions. If certain contaminants reach remote glaciers and accumulate on their surface in large quantities, it is reasonable to assume that their concentration is other ecosystems is significantly higher, which clearly indicates that current estimates are much too low.
The latest research carried out on glaciers in the Alps indicates that cryoconite constitutes a much better material for radioactivity studies in mountain and polar regions than lichens, mosses and peat deposits used so far. Research performed on Werenskiöldbreen and Hansbreen in Svalbard as well as on other glaciers in the Alps and the Caucasus showed that the activity of synthetic caesium, plutonium and americium on these glaciers is a few hundred times higher than in European soil, even in areas which received high amounts of radioactive fallout (such as Norway).
The work of Polish researchers has done a lot to boost awareness and stimulate international interest in extremely high radioactivity of glaciers and cryoconite. This has generated a range of crucial questions: Will radionuclides accumulated on glacier surface be gradually released as the glaciers melt? Is this a threat to nearby ecosystems? Can this be dangerous to humans? Research continues on an increasingly large scale, so we may soon get our answers.
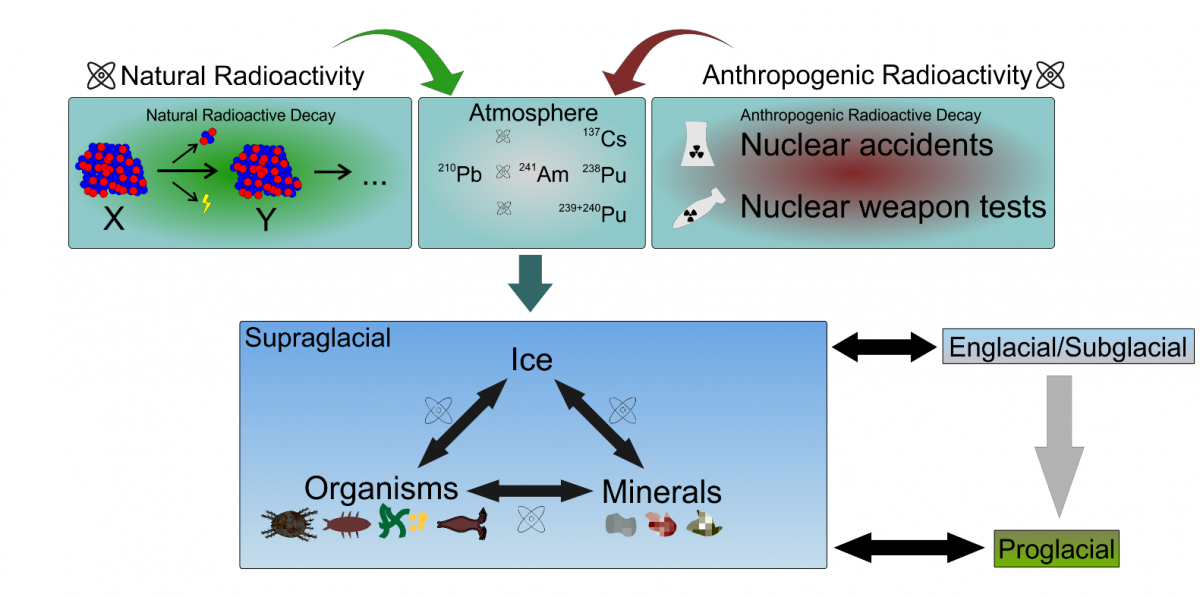
The diagram shows the main sources of artificial and natural radionuclides and their circulation in the glacier ecosystem.
Taxt: Dr. Krzysztof Zawierucha
Translation: Barbara Jóźwiak
Bibliography:
Baccolo, G., Łokas, E., Gaca, P., Massabò, D., Ambrosini, R., Azzoni, R.S., Clason, C., Di Mauro,
B., Franzetti, A., Nastasi, M., Prata, M., Prati, P., Previtali, E., Delmonte, B., Maggi, V., 2020. Cryoconite: an efficient accumulator of radioactive fallout in glacial environments. Cryosphere 14, 657–672.
Buda, J., Łokas, Ł., Pietryka, M., Richter, D., Magowski, W., Iakovenko, N.S., Porazinska, D.L., Budzik, T., Grabiec, M., Grzesiak, J., Klimaszyk, P., Gaca, P. & Zawierucha, K., 2020. Biotope and biocenosis of cryoconite hole ecosystems on Ecology Glacier in the maritime Antarctic. Sci. Total Environ, 724, 138112.
Jaworowski, Z., Kownacka, L., Grotowski, K., Kwiatkowski, K., 1978. LEAD-210 from nuclear explosions in the environment. Nucl. Technol. 37, 159–166.
Łokas, E., Mietelski, J.W., Ketterer, M.E., Kleszcz, K.,Wachniew, P., Michalska, S., Miecznik, M., 2013. Sources and vertical distribution of 137Cs, 238Pu, 239+240Pu and 241Am in peat profiles from Southwest Spitsbergen. Appl. Geochem. 28, 100–108.
Łokas, E., Zaborska, A., Kolicka, M., Różycki, M., Zawierucha, K., 2016. Accumulation of atmospheric radionuclides and heavy metals in cryoconite holes on an Arctic glacier. Chemosphere 160, 162–172.
Łokas, E., Zawierucha, K., Cwanek, A., Szufa, K., Gaca, P., Mietelski, J.W., Tomankiewicz, E., 2018. The sources of high airborne radioactivity in cryoconite holes from the Caucasus (Georgia). Sci. Rep. 8, 10802.






